Research
Full publication list on Pubmed, ORCID, ResearchGate, and Google Scholar
Entorhinal cortex represents task-relevant remote locations independent of CA1
Postdoctoral fellow in Dr. Lisa Giocomo’s lab at Stanford University, November 2019 - Present
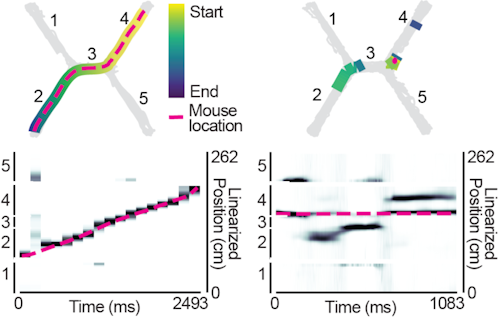
Neurons can collectively represent the current sensory experience while an animal is exploring its environment or remote experiences while the animal is immobile. These remote representations can reflect learned associations1–3 and be required for learning4. Neurons in the medial entorhinal cortex (MEC) reflect the animal’s current location during movement5, but little is known about what MEC neurons collectively represent during immobility. Here, we recorded thousands of neurons in superficial MEC and dorsal CA1 as mice learned to associate two pairs of rewarded locations. We found that during immobility, the MEC neural population frequently represented positions far from the animal’s location, which we defined as ‘non-local coding’. Cells with spatial firing fields at remote locations drove non-local coding, even as cells representing the current position remained active. While MEC non-local coding has been reported during sharp-wave ripples in downstream CA16, we observed non-local coding more often outside of ripples. In fact, CA1 activity was less coordinated with MEC during non-local coding. We further observed that non-local coding was pertinent to the task, as MEC preferentially represented remote task-relevant locations at appropriate times, while rarely representing task-irrelevant locations. Together, this work raises the possibility that MEC non-local coding could strengthen associations between locations independently from CA1.
Publications
- Aery Jones, E.A., Low, I.I.C., Cho, F.S., Giocomo, L.M. (2024, July). Entorhinal cortex represents task-relevant remote locations independent of CA1. bioRxiv.
- Masuda, F.K., Aery Jones, E.A.+, Sun, Y.+, Giocomo, L.M. (2023, October). Ketamine evoked disruption of entorhinal and hippocampal spatial maps. Nature Communications. (+equal contribution)
- Aery Jones, E.A. (2023, February). Chronic Recoverable Neuropixels in Mice. protocols.io.
- Aery Jones, E.A., Giocomo, L.M. (2023, February). Neural ensembles in navigation: from single cells to population codes. Current Opinion in Neurobiology.
- Video for lay audience explaining my research
Invited Talks
- April 2024: Dynamics of Hippocampal Inputs in Learning and Alzheimer’s Disease. Yale University Department of Neuroscience SYNAPSES seminar series in New Haven, CT.
- December 2023: Dynamics of Hippocampal Inputs in Learning and Alzheimer’s Disease. Stony Brook University Department of Neurobiology and Behavior BRITE Seminar Series in Stony Brook, NY.
- November 2023: Dynamics of Hippocampal Inputs in Learning and Alzheimer’s Disease. Georgetown University Department of Pharmacology and Physiology in Washington, DC.
- July 2023: Dynamics of Entorhinal Reactivations over Learning. Inhibition in the CNS Gordon Research Conference in Les Diablerets, Switzerland.
Posters
- November 2023: Dynamics of Entorhinal Reactivations over Learning. Society for Neuroscience Annual Meeting in Washington, DC.
- July 2023: Dynamics of Entorhinal Reactivations over Learning. Inhibition in the CNS Gordon Research Seminar and Conference in Les Diablerets, Switzerland.
- November 2022: Investigating How Medial Entorhinal Cortical Sequences Support Spatial Navigation and Learning. Society for Neuroscience Annual Meeting in San Diego, CA.
Sharp-wave ripple alterations mark memory decline and interneuron drive
PhD Student in Dr. Yadong Huang’s lab at the Gladstone Institutes & co-mentored by Dr. Loren Frank at University of California, San Francisco, January 2015 - October 2019
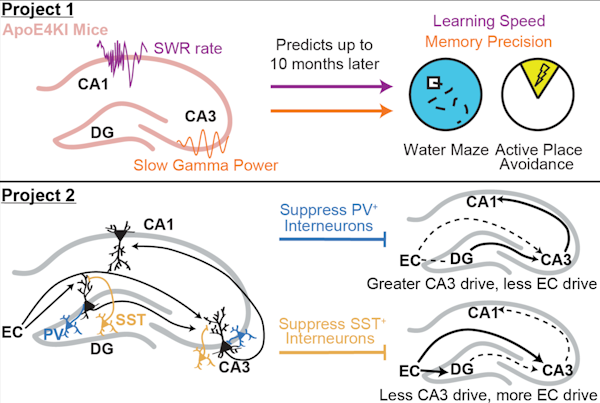
Hippocampal sharp-wave ripples (SWRs) – electrophysiological signatures of memory reactivation in the hippocampus – play an important role in memory processes. We tested the relationship between SWRs and memory impairment in an Alzheimer’s disease (AD) mouse model and the role of GABAergic interneurons in modulating SWRs. First, there is a pressing need to identify early pathophysiological alterations that predict subsequent memory impairment in AD. Mouse models of AD show reductions in both SWR abundance and associated slow gamma (SG) power during aging, suggesting SWRs may be a compelling candidate biomarker. In aged AD model mice, we found that reduced SWR abundance and associated CA3 SG power predicted spatial memory impairments measured 1–2 months later. Importantly, SWR-associated CA3 SG power reduction in young apoE4-KI mice also predicted spatial memory deficits measured 10 months later. Second, SWRs in CA1 are driven by inputs from upstream area CA3 and also engage the dentate gyrus (DG), but little is known about whether and how GABAergic interneurons in either CA3 or the DG regulate activity in CA1. The majority of hippocampal interneurons are parvalbumin-expressing (PV+), soma-targeting or somatostatin-expressing (SST+), distal dendrite-targeting subtypes, which are differentially impaired in AD. We find that that PV+ and SST+ interneurons bidirectionally modulate sleep SWRs in CA1 and coincident SG observed throughout the hippocampus. Overall, our results suggest that PV+ interneurons reduce CA3 coupling to CA1, while SST+ interneurons reduce entorhinal cortex coupling to CA1. These results establish features of SWRs as potential functional biomarkers of memory impairment in AD and probe how GABAergic interneuron subtypes impaired in AD modulate these SWR features.
- Thesis talk and slides
Publications
- PhD Thesis
- Taubena, D.R.+, Jang, S.-S.+, Grone, B.+, Yip, O., Aery Jones, E.A., Blumenfeld, J., Liang, Z., Koutsodendris, N., Rao, A., Ding, L., Zhang, A.R., Hao, Y., Xu, Q., Yoon, S.Y., Huang, Y. (2024, June). Neuronal APOE4-induced Early Hippocampal Network Hyperexcitability in Alzheimer’s Disease Pathogenesis. bioRxiv. (+equal contribution)
- Santiago, R.M.M., Lopes-dos-Santos, V., Aery Jones, E.A., Huang, Y., Dupret, D., Tort, A.B.L. (2024, February). Waveform-based classification of dentate spikes. Scientific Reports.
- Aery Jones, E. A., Rao, A., Zilberter, M., Djukic, B., Bant, J.S., Gillespie, A. K., Koutsodendris, N., Nelson, M., Yoon, S. Y., Huang, K., Yuan, H., Gill, T. M., Huang, Y., & Frank, L. M. (2021, December). Dentate Gyrus and CA3 GABAergic Interneurons Bidirectionally Modulate Signatures of Internal and External Drive to CA1. Cell Reports.
- Taubes, A.T., Nova, P., Zalocusky, K.A., Kosti, I., Bicak, M., Zilberter, M., Hao, Y., Yoon, S.Y., Oskotsky, T., Pineda, S., Chen, B., Aery Jones, E.A., Choudhary, K., Grone, B., Balestra, M.E., Chaudhry, F., Paranjpe, I., De Frietas, J., Koutsodendris, N., Chen, N., Wang, C., Chang, W., An, A., Glicksberg, B., Sirota, M., Huang, Y. (2021, October) Experimental and real-world evidence supporting the computational repurposing of bumetanide for APOE4-related Alzheimer’s disease. Nature Aging.
- Najm, R., Zalocusky, K.A., Zilberter, M., Yoon, S.Y., Hao, Y., Taubes, A., Jones, E. A., Koutsodendris, N., Nelson, M., Rao, A., Huang, Y. (2020, July). In Vivo Chimeric Alzheimer’s Disease Modeling of Apolipoprotein E4 Toxicity in Human Neurons. Cell Reports.
- Jones, E. A., Gillespie, A. K., Yoon, S. Y., Frank, L. M., Huang, Y. (2019, November). Early Hippocampal Sharp-Wave Ripple Deficits Predict Later Learning and Memory Impairments in an Alzheimer’s Disease Mouse Model. Cell Reports.
- Najm, R.+, Jones, E. A.+ & Huang, Y. (2019, June) Apolipoprotein E4, Inhibitory Network Dysfunction, and Alzheimer’s Disease. Molecular Neurodegeneration. (+equal contribution)
- Gillespie, A. K., Jones, E. A. & Huang, Y. (2017, February) Approaching Alzheimer’s Disease from a Network Level. Oncotarget.
- Gillespie, A. K., Jones, E. A., Lin, Y.-H., Karlsson, M. P., Kay, K., Yoon, S. Y., Tong, L. M., Nova, P., Carr, J. S., Frank, L. M., Huang, Y. (2016, May). Apolipoprotein E4 causes age-dependent disruption of slow gamma oscillations during hippocampal sharp-wave ripples. Neuron.
Invited Talks
- July 2019: Hippocampal GABAergic Interneurons Bidirectionally Modulate Sharp-Wave Ripples. Inhibition in the CNS Gordon Research Seminar in Newry, MA.
- April 2019: Ripple Deficits Predict Memory Impairments in an Alzheimer’s Disease Mouse Model. Discovery Fellows Michael Page Research Symposium in San Francisco, CA.
- August 2018: Optogenetic Study of ApoE4-Related Alzheimer’s Disease. NIA Optogenetics RFA Annual Investigators Meeting in Bethesda, MD.
- September 2017: Apolipoprotein E4-induced Hippocampal Network Activity Deficits Reflect Cell-Type-Specific Gains of Toxic Function. Alzheimer’s Researcher Symposium in San Francisco, CA.
- August 2017: Optogenetic Study of ApoE4-Related Alzheimer’s Disease. NIA Optogenetics RFA Annual Investigators Meeting in Bethesda, MD.
- June 2017: Apolipoprotein E4-induced Hippocampal Network Activity Deficits Reflect Cell-Type-Specific Gains of Toxic Function. Gladstone Institutes Scientific Retreat, Asilomar, CA.
Posters
- July 2019: Hippocampal GABAergic Interneurons Bidirectionally Modulate Sharp-Wave Ripples. Inhibition in the CNS Gordon Research Seminar in Newry, MA.
- November 2018: Apolipoprotein E4-induced Hippocampal Network Activity Deficits Correlate with Learning and Memory Impairments. Society for Neuroscience Annual Meeting in San Diego, CA.
- June 2018: Apolipoprotein E4-induced Hippocampal Network Activity Deficits Correlate with Learning and Memory Impairments. Advances in Neurodegenerative Research and Therapies Keystone Symposium in Keystone, CO.
- November 2017: Apolipoprotein E4-induced Hippocampal Network Activity Deficits Reflect Cell-Type-Specific Gains of Toxic Function. Society for Neuroscience Annual Meeting in Washington, D.C.
- June 2017: Apolipoprotein E4-induced Hippocampal Network Activity Deficits Reflect Cell-Type-Specific Gains of Toxic Function. Inhibition in the CNS Gordon Research Conference, Les Diablerets, Switzerland.
Natural selection of intrinsic disorder characteristic of proteins
Undergraduate Research Assistant in Dr. Sridhar Hannenhalli’s lab at the University of Maryland Center for Bioinformatics and Computational Biology, May 2013 - August 2014
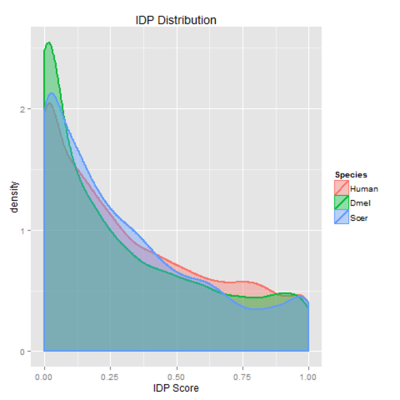
Intrinsically disordered proteins lack stable tertiary structure, are recent in molecular evolution, and are highly regulated due to their low affinity for their substrates. This disorder is selected for even when the underlying sequence is not conserved; thus, I developed a new method of modeling in silico evolution which measures selection for protein properties not directly encoded in the sequence. My algorithm used protein orthologs to create synthetic proteins using the same parameters as in vivo sequence evolution, then measured purifying selection for disorder by comparing selected mutations to non-selected ones. The resulting selection showed enrichment for disorder in particular between less conserved sequences. This algorithm could be used in the future to measuring purifying selection of other protein properties not directly encoded in the sequence, such as overall charge or polarity. Moreover, since proteins with high intrinsic disorder are enriched in cell signaling, transcription, and chromatin remodeling, identifying proteins with high selective pressures could help detect novel proteins involved in cancer and neurodegenerative diseases.
Posters
- November 2013: Natural Selection of Intrinsic Disorder Characteristic of Proteins. University of Maryland Bioscience Research Day, College Park, MD.
The impact of prenatal nicotine exposure on impulsivity and neural firing in the medial prefrontal cortex
Gemstone Honors Program Member of Team RITALIN in Dr. Matthew Roesch’s lab at the University of Maryland Department of Psychology, May 2011 - May 2014
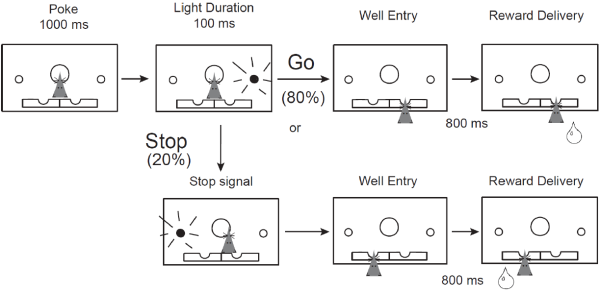
Behavioral, neurochemical, and neuroanatomical disturbances following prenatal nicotine exposure (PNE) suggest that PNE could serve as a model of ADHD, a disorder which has not been well-characterized in animal models. To confirm the face and construct validity of this model, we measured single-unit neuronal firing in the medial prefrontal cortex (mPFC) in PNE rats during the stop-signal task, which measures inhibition of an already-initiated action. Consistent with our hypothesis, we found that PNE rats were faster and more impulsive, and that mPFC activity was modulated by trial direction and type. PNE mPFC neurons were overall hypoactive as compared to controls, but the directional encoding was not affected. This suggests that reduced firing in the mPFC may promote impulsive behavior and that general increases in mPFC activity might rescue the deficits observed following PNE. Our research is the first connection between an environmental cause of ADHD, impulsivity symptoms, and the underlying neural firing patterns. Thus, we discovered PNE rats could be a unique model of this multi-faceted disorder.
- Thesis talk: Barnett, B. R., Cohen, V. J., Hearn, T. N., Jones, E. A., Kariyil, R. J., Kunin, A., Kwak, S. I., Lee, J., Lubinski, B. L., Rao, G. K., Zhan, A. (2014, April). The Impact of Prenatal Nicotine Exposure on Impulsivity and Neural Firing in the Medial Prefrontal Cortex
Publications
- Thesis
- Bryden, D. W., Burton, A. C., Barnett, B. R., Cohen, V. J., Hearn, T. N., Jones, E. A., Kariyil, R. J., Kunin, A., Kwak, S. I., Lee, J., Lubinski, B. L., Rao, G. K., Zhan, A., Roesch, M. R. (2016, February). Prenatal Nicotine Exposure Impairs Executive Control Signals in Medial Prefrontal Cortex. Neuropsychopharmacology
Posters
- November 2013: Impact of Prenatal Nicotine Exposure on Impulsivity and Neural Acitivity in Medial Prefrontal Cortex. Society for Neuroscience Annual Meeting, San Diego, CA.
- May 2013: Validating an Animal Model of Attention Deficit Hyperactivity Disorder: Neural and Behavioral Correlates of Impulsivity in Rats Prenatally Exposed to Nicotine. University of Maryland Undergraduate Research Day, College Park, MD.
- March 2013: Validating an Animal Model of Attention Deficit Hyperactivity Disorder: Neural and Behavioral Correlates of Impulsivity in Rats Prenatally Exposed to Nicotine. Howard Hughes Medical Institute (HHMI) Undergraduate Research Symposium, College Park, MD.
Western blot semi-quantitative analysis of non-canonical cAMP-dependent protein expression induced by PACAP
Montgomery Blair Magnet Program Senior Research Project in Dr. Lee Eident’s lab at the National Institute of Mental Health, June 2009 - August 2009
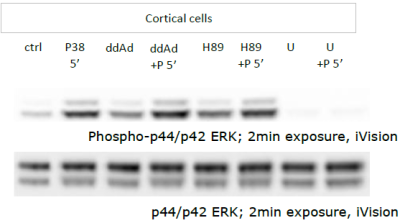
Publications
- Senior thesis
- I am not an author on any publications from this research. However, I contributed data collection and analysis to two papers: Holighaus et al, 2011 and Holighaus et al, 2012
Posters
- April 2010: Western Blot Semi-Quantitative Analysis of Non-Canonical cAMP-Dependent Protein Expression Induced by PACAP. Blair Magnet Senior Research Symposium in Silver Spring, MD.
